Chapter : 4. Structure Of Atoms
Neutron
In 1932, Chadwick discovered the fundamental particle neutron. The neutron is a neutral particle found in the nucleus of an atom. The subatomic particle not present in a hydrogen atom is neutron. A hydrogen atom contains only one proton and one electron. A neutron is represented by the symbol n.
Characteristics of a Neutron
Mass of a neutron : The mass of neutron is equal to the mass of a proton. The relative mass of a neutron is 1 u. The absolute mass of a neutron is 1.6 × 10–27 kg.
Charge of an neutron : Neutron has no charge. It is electrically neutral.
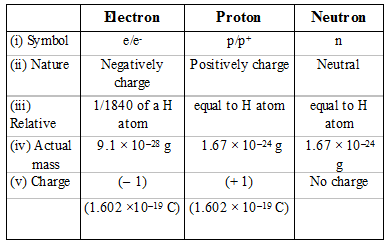
Comparision between Proton, Neutron and Electron:
Characteristics of a Neutron
Mass of a neutron : The mass of neutron is equal to the mass of a proton. The relative mass of a neutron is 1 u. The absolute mass of a neutron is 1.6 × 10–27 kg.
Charge of an neutron : Neutron has no charge. It is electrically neutral.
Comparision between Proton, Neutron and Electron:

Trending Articles & Blogs
- Physics Tutor, Math Tutor Improve Your Child’s Knowledge
- How to Get Maximum Marks in Examination Preparation Strategy by Dr. Mukesh Shrimali
- 5 Important Tips To Personal Development Apply In Your Daily Life
- Breaking the Barriers Between High School and Higher Education
- 14 Vocational courses after class 12th
- Tips to Get Maximum Marks in Physics Examination
- Get Full Marks in Biology Class 12 CBSE
Download Old Sample Papers For Class X & XII
Download Practical Solutions of Chemistry and Physics for Class 12 with Solutions
Recent Questions Asked
- Newton’s laws of motion asked by Dr. Mukesh Shrimali
- Process of nutrition in Amoeba asked by Rajiv Sharma
- Importance of studying physics subject in school after 10th asked by Rajiv
- Refraction Through Prism in Different Medium asked by Kirti Sharma
- Ratio and Proportion Question asked by Education Desk
- Explain all the 12 tenses with example asked by Qwerty
- Refraction Through Prism in Different Medium asked by Seema Shrimali